Hgo Hg O2 Redox

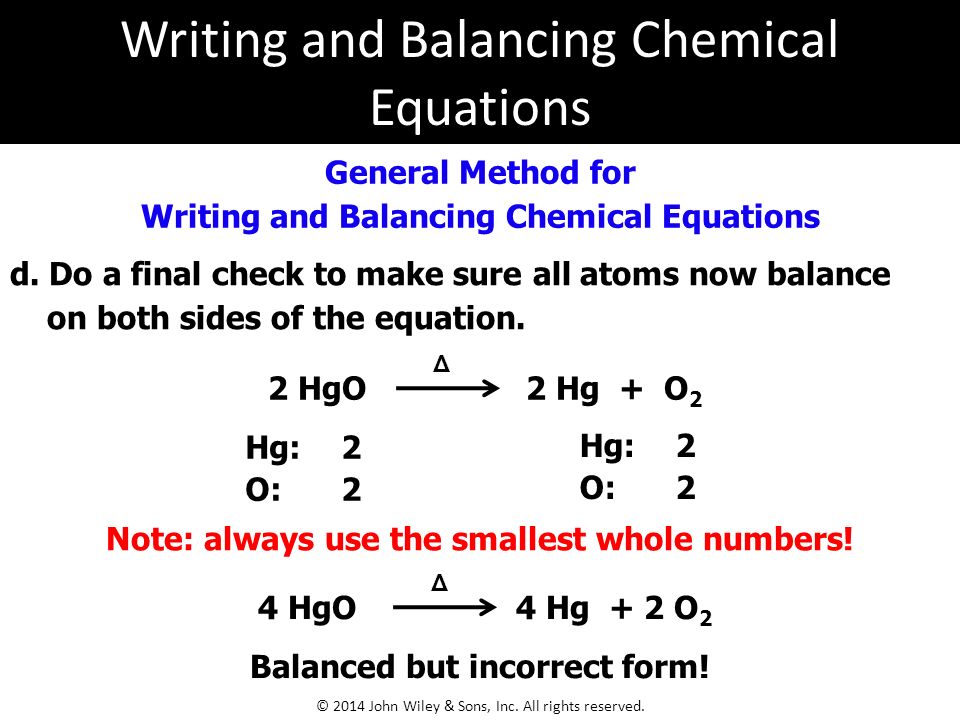
2 hgo 2 hg o2 decomposition 6.
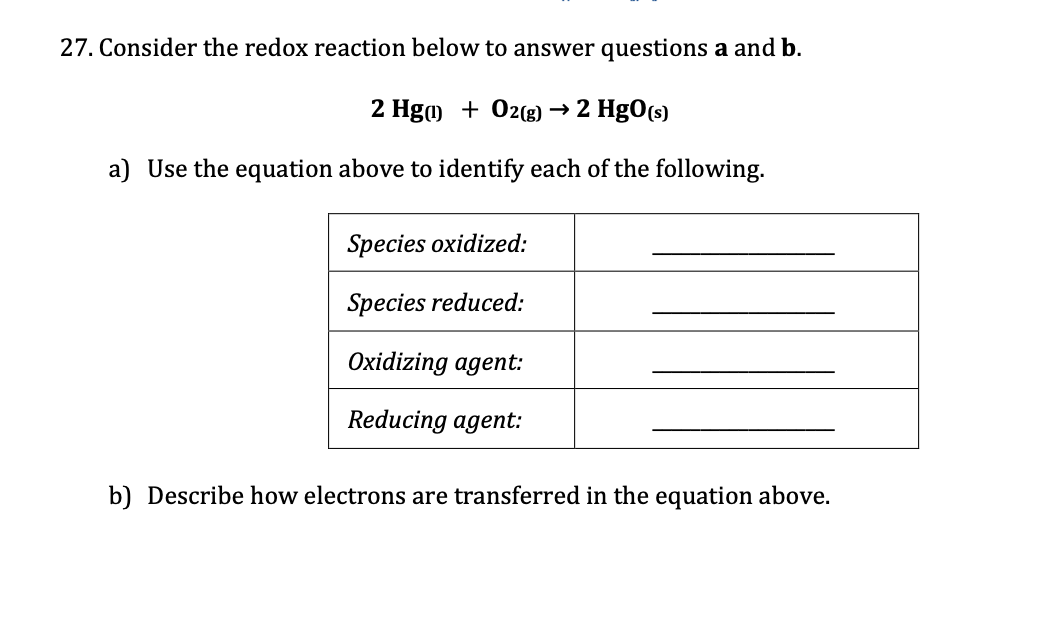
Hgo hg o2 redox. Cu 2 agno3 cu no3 2 2 ag single replacement 8. Characteristics of redox reaction oxidation reduction reaction takes. Hgo hg o2 chemical equation balancer. H in c2h6 has a 1 oxidation state.
And it s a redox reaction. So c went from 3 to 4 and was oxidized and o went from 0 to 2 and was reduced. 5 is the. H2so4 zn znso4 h2 balanced.
The answer will appear below. H2so4 zn znso4 h2 single replacement 7. Hgo hg o2 redox ju 0caco3 cat co2 not redox hgo hg o2 redox ju 0caco3 cat co2 not redox. The book parsing techniques a practical guide imho one of the best computer science books ever written.
H in h2o is 1. O in o2 has a 0 oxidation state. Reduction is defined as a reduction in charge. View notes oxidizing reducing agents pdf from chm 1025c at valencia community college.
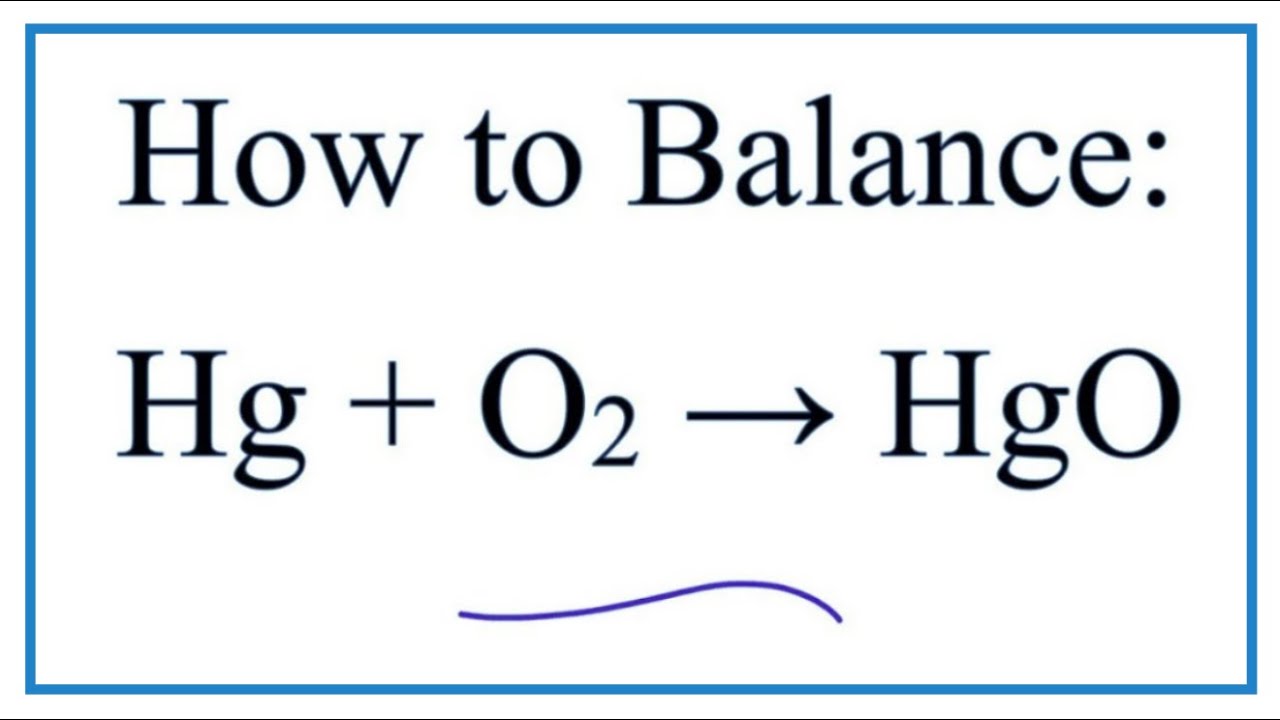
O in h2o is 2. Molar mass of hgo bond polarity of hgo oxidation state of hgo. In order to balance the chemical equation hgo hg o2 watch this video till end and you ll surely understand how to balance it. To balance hg o2 hgo you will need to be.
Enter an equation of a chemical reaction and click balance. Redox reaction a chemical reaction in which oxidation and reduction reaction takes place simultaneously is known as redox reaction. C in co2 is 4. In this video we will balance the equation hg o2 hgo and provide the correct coefficients for each compound.
2 hgo 2 hg o 2. Always use the upper case for the first character in the element name and the lower case for the second character. Hgo hg o2 balanced. This program was created with a lot of help from.
O in co2 is 2. The gold parsing system hats off. Explanation this is an oxidation reduction redox reaction ca0 2 e caii oxidation 2 o0 2 e 2 o i reduction ca is a reducing agent o2 is an oxidizing. Hgo hg o2 hgois undergoing reduction to hg by the removal of electronegative oxygen atom.
Cu agno3 cu no3 2 ag balanced. Gain of electron is reduction. Instructions on balancing chemical equations. Mg2 2e mg.
Mercury ii oxide hgo. C in c2h6 has a 3 oxidation state.